Bladder Cancer
The prognosis for bladder cancer has been relatively unchanged over the last decades, but patient treatment has become gentler for the different types of intervention. However, in general it is found that there is much to be gained in regard to diagnostics, treatment and sequelae.
The objective of research into bladder cancer and bladder tumours at the Research Unit at the Department of Urology is to cover all aspects of the disease from the smallest papilloma to advanced bladder cancer with metastases, as well as from early detection over molecular medicine methods to rehabilitation following major surgery.
The research unit wishes to be among the world leaders in the field of bladder cancer research and a leader in new treatment methods.
As a general principle, patients with bladder tumours who are treated at the Department of Urology should be offered participation in a minimum of one research project if they so wish, as this enables the continuous development of the area for the benefit of the both the individual and future patients.
Project information:
For detailed information on our projects and the bladder cancer research team please visit:
Projects
Non-Muscle-Invasive Bladder Cancer
Diagnosis
BC-DEEDS Study - Bladder Cancer Deep Learning Diagnostics Study
Title
BC-DEEDS Study – Bladder Cancer Deep Learning Diagnostics Study. – Can a deep learning tool improve diagnostics in patients with bladder cancer?
Aim
As bladder cancer has a high recurrence and progression rate knowledge of tumor grade is essential to perform an optimal resection. However, visual estimation by the surgeon regarding tumor grade is poorly correlated to the true histopathological tumor grade, which is the main reason for the high recurrence rate and delay of relevant adjuvant treatment.
The project aim at training a Convolutional Neural Network (CNN) to improve detection of bladder lesions and assist in the prediction of the histopathological diagnose.
By using a CNN-model to classify bladder lesions and predicting tumor grade the vision for the project is to improve the overall management of patients with NMIBC.
Number of patients
3200 patients
Methods
A prospective study where video data and images from flexible cystoscopy or TUR-B will be used to produce a trained CNN-model, which can be implemented in a software-application to detect various bladder lesions. Data on non-muscle invasive bladder cancer (NMIBC), muscle invasive bladder cancer (MIBC), Carcinoma In Situ (CIS), inflammation, cystitis cystica and normal bladder mucosa is included in the data set.
Participants for this study are patients eligible for cystoscopy or TUR-B. There will be no changes in regular procedure for these patients, nor in the follow up of their disease.
Status
Ongoing
Sites
Department of Urology at Aarhus University Hospital and Regional Hospital of Holstebro
Contacts
Professor and chair, Jørgen Bjerggaard Jensen, email: bjerregaard@skejby.rm.dk
Clinical Trial Coordinator, Michael P. Hjørnholm, email: micott@rm.dk
GreenBladder
Title: GreenBladder – Early detection of bladder cancer in residents in Greenland using a urine marker and a mobile cystoscopy unit
Aim: The GreenBladder study aims at screening all adults more than 40 years old in Greenland for bladder cancer to detect patients with early potentially curable tumor stages. Following treatment, urinary marker follow-up will be used to facilitate regular follow-up in locations with limited availability of urological service. With the early detection and meticulous follow-up, we aim at improving the current very poor prognosis for bladder cancer patients in Greenland.
Number of patients: All citizens of Greenland above the age of 40 years, living in the 13 towns with more than 1.000 citizens, will be invited to a screening visit at the local hospital or health clinic.
Methods:
The screening visit consist of:
• Interview regarding visible hematuria within the last year.
• A short questionnaire on demographics and potential risk factors for development of bladder cancer.
• Urine dipstick.
• Xpert ® Bladder Cancer Detection test.
If the person investigated fulfills any of the criteria below, he/she will be offered a PrimeSight flexible cystoscopy:
• Visible hematuria within the last year.
• Positive Xpert ® Bladder Cancer Detection test.
Status: We expect to be able to start up in the spring of 2023 and implement our screening visits during 2023-2024 in the 13 cities.
Sites:
Queen Ingrid´s Hospital
Jens Kreutzmannip aqq 11.
Postbox 1001
3900 Nuuk
Tasiilaq Health Center
Postboks 510
3913 Tasiilaq
Narsaq Health Center
Postbox 32
3921 Narsaq
Qaqortoq Regional Hospital
Postbox 512
3920 Qaqortoq
Paamiut Health Center
Postbox 98
3940 Paamiut
Nanortalik Hospital
Box 169
3922 Nanortalik
Maniitsoq Health Center
Postbox 501
3912 Maniitsoq
Sisimiut Region Hospital
Postbox 1013
3911 Sisimiut
Aassiaat Region Hospital
Postbox 212
3950 Aasiaat
Qasigiannguit Health Center
Postbox 114
3951 Qasigiannguit
Ilulissat Regional Hospital
Postbox 514
3952 Ilulissat
Uummannaq Health Center
Postbox 191
3961 Uummannaq
Upernavik Health Center
Postbox 64
3962 Upernavik
Contacts:
Ph.D. student, Nathalie Demuth Fryd, email: NATFRY@rm.dk
Clinical Trial Coordinator, Tine Christiansen, email: tinechti@rm.dk, Phone: + 45 30 91 54 59
Treatment
Optimal approach for T1 bladder cancer - Clinical relevance of subclassification in T1 bladder cancer
Titel: Optimal approach for T1 bladder cancer – Clinical relevance of subclassification in T1 bladder cancer
Background
Bladder Cancer (BC) is the 10th most commonly diagnosed cancer worldwide. Approx. 450 patients are diagnosed with tumour stage T1 BC in Denmark (DK) each year. In DK, the 5-year overall- and cancer specific survival (CSS) after the diagnosis of T1 BC is approx. 60% and 80%, respectively.
In most industrialized countries, T1 BC is treated with tumour resection and BCG bladder instillation. However, the 5- and 10-year risk of progression to muscle invasive BC (MIBC) with this approach is 31% and 42% respectively. In addition, patients that progress from T1 BC to MIBC have a 5-year CSS of < 50% compared 67% for patients with primary MIBC.
In DK T1 BC is sub classified into T1a and T1b. Most T1a patients will receive tumour resection and BCG instillation and most T1b patients will receive surgical removal of the urinary bladder.
No studies have compared the Danish approach with the more conservative and widespread bladder preserving approach.
Aim
Study 1: To investigate the prognostic value of T1 BC sub classification.
Study 2: To compare the prognosis of T1 BC patients diagnosed in DK and Sweden.
Study 3: To investigate the reproducibility and the clinical relevance of a new pathological definition of the T1 BC sub classification.
Methods
Study 1: Danish national data on T1 BC patients diagnosed from Sept. 2012 to Aug. 2018 will be retrieved. Data on T1a and T1b patients will be compared.
Study 2: For the same period as in study 1, data on T1 BC patients from DK will be compared with data on T1 BC patients for Sweden.
Study 3: For the same period as in study 1, pathological tissues from Danish T1 BC patients will get re-evaluated according to a new definition.
Statistical analysis: In all three studies overall-, cancer specific-, recurrence free- and progression free survival will be compared using cox proportional hazard regression analysis stratified on treatment modality and adjusted for gender, age and comorbidity. For study 3, interrater agreement will be calculated using Cohen´s Kappa.
Perspective
The study will evaluate whether T1 subclass. in T1a and T1b should be recommended on an international level with potential positive effect on oncological outcomes. In addition, the results from this study are expected to contribute with updated knowledge on the treatment of T1 BC. Hereby to provide cancer urologists with updated knowledge to allow optimal guidance of T1 BC patients regarding treatment of their BC disease.
Contact
Phd student: Erik Hansen
Main supervisor: Jørgen Bjerggaard Jensen
En Bloc
Title: En Bloc Transurethral Resection Of Non-Muscle Invasive Bladder Cancer
Bladder cancer is the tenth most common cancer in the world, and approximately 75% present with non-muscle invasive bladder cancer (NMIBC). The cornerstone in diagnosis and treatment of bladder tumours is transurethral resection (TURB) where the tumour is dissected in pieces, removed from the bladder, and then pathologically examined to identify potential detrusor muscle invasion. This method leads to two problems: first, incomplete resection of tumour and possible scattering of tumour cells may lead to recurrence. Second, pathological examination is impaired since tumour margins cannot be properly assessed. Thus, infiltration of suburothelial tissue or detrusor muscle may be underestimated or even missed.
En bloc resection (EBR), where the tumour is removed in total, can potentially overcome the above mentioned flaws of conventional TURB.
We are conducting a randomised controlled multicentre trial comparing EBR to conventional TURB in patients with non-muscle invasive bladder cancer. We hypothesize EBR to be a superior operation technique to conventional TURB regarding complete tumour resection and pathological assessment quality.
Patients with primary NMIBC tumours with diameter ≥1cm and <6cm are randomised to either the intervention group, undergoing EBR, or the control group, undergoing cTURB. Sample size will be 220 patients in total, 110 in each group. Ultimately, a central pathology revision of all specimens is performed to investigate the pathological outcome of EBR compared to cTURB. The primary endpoint will be number of patients with an unaltered T-stage at pathology revision compared to the initial resection and potential second resection.
This study can evaluate the ability of En Bloc-resection to remove NMIBC tumours with better pathological quality and certainty compared with cTURB. Positive results could potentially redefine guidelines on golden standard for preferred resection technique in future NMIBC patients.
Contact:
Phd student: Ninna Kjær Nielsen
Main supervisor: Jørgen Bjerggaard Jensen
The EpiCheck Trial
Aim
To evaluate whether EpiCheck can be used as a predictor of tumour response to short-term intensive chemoresection with mitomycin C
Number of patients
22
Methods
The Bladder Epicheck® test is a diagnostic device for the detection of urinary DNA methylation-patterns associated with bladder cancer. DNA methylation is an epigenetic modification often involved in bladder cancer. The EpiCheck test is based on analysis of 15 DNA methylation biomarkers, of which the EpiCheck software calculates a methylation score. The score ranges from 0-100 representing the overall methylation level from low to high. A score above 60 is considered positive and corresponding to a high risk of recurrent bladder cancer.
The study is a prospective observational study without randomisation. Eligible patients; have a history of Ta high-grade bladder tumours, are diagnosed with a recurrence and referred to short-term intensive chemoresection with mitomycin C. The treatment adhere to the regimen described in the NICSA and DaBlaCa-13 trial. Treatment consists of short-term, intensive chemoresection with mitomycin C thrice weekly for two weeks. To evaluate tumour response, an early cystoscopy is performed in the outpatient clinic four weeks after treatment completion. Subsequently patients continue a standardised follow-up program assuming Danish guidelines, which is initiated after fourth months. The EpiCheck test is performed at baseline and if the result is positive, the test is repeated prior to the fourth and sixth instillation with mitomycin C as well as prior to the subsequent two cystoscopies. Urine cytology is performed simultaneously with the EpiCheck test. Clinicians are blinded to the EpiCheck result.
Status
Patient inclusion is ongoing with four patients included.
Sites
- Department of Urology, Aarhus University Hospital, Aarhus, Denmark
- Department of Molecular Medicine, Aarhus University Hospital, Aarhus, Denmark
Contacts
Phd student: Ninna Kjær Nielsen, ninna.nielsen@rm.dk
North-Reg Dwell Time Study
Title: North-Reg Dwell Time Study
Aim
The aim of this study is to investigate whether reduced dwell time (DT), being the time the bladder is exposed to Bacillus Calmette Guérin (BCG), will decrease the severity of side effects due to BCG instillations compared to the current DT at 2 hours.
Previous studies show that approximately 70% of non-muscle invasive bladder cancer patients experience side effects when treated with BCG. When installed in the bladder BCG causes an immune response killing the bladder cancer cells. Because of the side effects, patients terminate their instillations before completing all planned instillations, resulting in an increased risk of recurrence and progression.
Number of patients
314
Methods
The trial is designed as a multi-center, multi-national, two-armed, randomized, open-label, investigator-initiated clinical controlled – phase IV trial.
To monitor the patients side effects they will receive daily questionnaires in the instillation weeks. The side effects will be categorized according to a predefined algorithm and if patients in the intervention arm experience side effects of a certain severity, they will be reduced in DT.
Status
Recruiting in all sites (- Rigshospitalet, Copenhagen University Hospital, Denmark)
Sites
1. Department of Urology, Aarhus University Hospital, Denmark
2. Department of Urology, Herlev University Hospital, Denmark
3. Department of Urology, Roskilde Regional Hospital, Denmark
4. Department of Urology, Aalborg University Hospital, Denmark
5. Department of Urology, Holstebro Regional Hospital, Denmark
6. Department of Urology, Rigshospitalet, Copenhagen University Hospital, Denmark
7. Department of Urology, Odense University Hospital, Denmark
8. Department of Urology, Örebro Regional Hospital, Sweden
9. Department of Urology, PO Salgrenska University Hospital, Sweden
10. Department of Urology, Landspitalinn University Hospital, Iceland
11. Department of Urology, Karolinska University Hospital, Sweden
Contacts
PhD student, MD, Lene Munk, lenemu@rm.dk
OPTIMA – Outpatient laser ablation of large recurrent non-muscle invasive bladder cancer
Title: Outpatient laser ablation of large recurrent non-muscle invasive bladder cancer – a prospective feasibility study – OPTIMA
Aim
To ivestigate the feasibility and tolerability of the Olympus Soltive™ laser in treatment of large recurrent low-grade Ta tumours in an outpatient setting.
Number of patients
145
Methods
This project is a prospective feasibility study. Included patients will undergo outpatient laser ablation using the Olympus Soltive™ laser under local anaesthetics. Participants will be contacted on day 1 and 14 following the procedure in order to assess satisfaction. Primary endpoint is the proportion of successful laser ablation outpatient procedures compared to failed procedures leading to standard TURBT. Secondary endpoints include median VAS score and time to recurrence.
Status
Commencing in spring 2022
Sites:
Department of Urology, Aarhus University Hospital, Denmark
Department of Urology, Fundació Puigvert, Universitat Autònoma de Barcelona, Spain
Department of Urology, Motol University Hospital, Czech Republic
Department of Urology, Institut Universitaire du Cancer Toulouse Oncopole, France
Contact
Ph.d. student Vanaja Kumarasegaram, email: vanakuma@rm.dk
Clinical Trial Coordinator, Vibeke Juul Morrison, email: vimoor@rm.dk
Follow-Up
SEALS Xpert - Surveillance of High-grade Non-muscle Invasive Bladder Tumours Using the Xpert Bladder Cancer Monitor -DaBlaCa 15
Surveillance Of High-Grade Non-Muscle Invasive Bladder Tumours Using The Xpert Bladder Cancer Monitor – SEALS Xpert
Title: Surveillance of high grade non-muscle invasive bladder tumours using the Xpert Bladder Cancer Monitor – SEALS
Aim
To investigate the feasibility of the Xpert Bladder Cancer Monitor as a substitute for cystoscopy in the follow-up of non-muscle invasive bladder cancer.
Number of patients
392 patients needed for 2 arms.
Methods
Multicenter, non-inferiority randomized clinical trial
Status
Ongoing
Sites
Regionshospital Gødstrup, Zeeland University Hospital at Roskilde, Aalborg University Hospital and Aarhus University Hospital
Contacts:
Primary investigator: MD, Ph.d. Thomas Karmark Dreyer thomas.dreyer@rm.dk
Uromonitor - Surveillance of low-grade non-muscle invasive bladder tumours using Uromonitor
Title: Surveillance of low-grade non-muscle invasive bladder tumours using Uromonitor – SOLUSION Uromonitor
Aim
The study aim at evaluating the potential clinical impact of the Uromonitor-test regarding possible reduction in number of cystoscopies, without increasing the risk of progression.
Number of patients
160 subjects
Methods
Uromonitor’ is an non-invasive urine based IVD diagnostic test.
The study is designed as a multicenter observational clinical trial. Patients scheduled for follow-up cystoscopy due to previous low grade NMIBC disease within the first 2 years from diagnosis, where no recurrence is found at cystoscopy, will be offered inclusion in the study.
Cystoscopy and Uromonitor test is performed at the time of inclusion. Patients with negative Uromonitor test and no visualization of tumor will be scheduled for a Uromonitor test every 4 months and Uromonitor test and cystoscopy after 8 and 24 months from the time of diagnosis for newly diagnosied patientes and after 12 and 24 months from the time of inclusion for perviousely diagnosied patients. Patients with positive Uromonitor test and no visualization of tumor at cystoscopy will be scheduled for cystoscopy as standard follow up without additional intervention. Recurrent tumors detected by cystoscopy will be handled according to current Danish guidelines.
Status
Recruiting at all sites
Sites in Denmark
Department of Urology, Zealand University Hospital, Roskilde
Department of Urology, Aarhus University Hospital
Contacts:
Thomas Karmark Dreyer, MD Sub-PI, thmacris@rm.dk
Professor Jørgen Bjerggaard Jensen, Bjerggaard@skejby.rm.dk
CONQUER-score - Complications' impact ON QUality of life after treatment for bladder cancER
Title: Complications impact oN QUality of life after treatment for non-muscle invasive bladder cancER (CONQUER-score)
Aim
To develop and validate a symptom-based scoring system for urinary dysfunction after treatment for non-muscle invasive bladder cancer (NMIBC)
Number of patients
We expect to include 1000 patients from the cross-sectional study
Methods
For this study we will develop and validate a scoring system for urinary, sexual and bowel dysfunction after treatment. The primary draft of the questionnaire for the score will be based on a thorough review of the literature and assessment by an expert panel. Questionnaires regarding symptoms and QoL will be sent to all eligible patient identified in the The National Patient Registry (LPR). Patients are eligible if they are above the age of 18 years, have a primary bladder cancer pTa or pT1a and are treated with TUR-B. Associations between items and QoL will be computed by binomial regression analyses. The important/significant items will be selected and regression analysis will be performed in order to find the adjusted risk ratios. Individual score values will be designated items to form the symptom-score, dividing the outcome into “no impact,” “minor impact,” and “major impact on QoL”. Validity will be tested by receiver operating characteristic (ROC) curve and Spearman’s rank correlation and discriminant validity will be tested by Student t tests.
Sites
This study is a national study, inviting patients treated at all sites in Denmark.
Contacts
PhD student Rikke Milling, rimill@rm.dk
Muscle-Invasive Bladder Cancer
Etiology
Influence of Hormone Treatment in Bladder Cancer – incidence, prognosis and functional outcome
Title: Influence of Hormone Treatment in Bladder Cancer – incidence, prognosis and functional outcome
Aim
To investigate the role of sex hormone receptors in the bladder and their potential role in the gender difference seen in the disease. Furthermore, this study aims to investigate if anti-hormonal treatment targeting these receptors is useful in the treatment for bladder cancer.
Number of patients
100.000
Methods
Register-based follow up cohort study. Patients are included from Danish National Registries. Patients diagnosed with prostate cancer, breast cancer or endometrial are included as cases and matched with a control group without cancer. Primary endpoint is bladder cancer diagnosis. Furthermore, patients are divided into sub-groups depending on the type of anti-hormonal treatment they receive.
Status
Ongoing
Sites
Department of Urology, Aarhus University Hospital
Contacts
PhD Student Josephine Hyldgaard, email: josehyld@rm.dk
Diagnosis
MAINTAIN - Residual tumor and complete local response to neoadjuvant chemotherapy in patients with muscle invasive bladder cancer evaluated by 15O-H2O PET/MR
Title: Residual tumor and complete local response to neoadjuvant chemotherapy in patients with muscle invasive bladder cancer evaluated by 15O-H2O PET/MR – MAINTAIN
Aim
The study aim is to investigate if 15O-H2O PET/MR can predict residual tumor and complete response to neoadjuvant chemotherapy in patients with MIBC and thereby identify potential candidates for organ preservation.
Number of patients
54
Methods
Patients scheduled for NAC followed by radical cystectomy due to histologically documented MIBC stage cT2-4a in the urinary bladder will be included. A 15O-H2O PET/MR scan will be performed at time of inclusion (baseline) and again after NAC prior to cystectomy.
Status
inclusion is ingoing
Sites
Aarhus University Hospital
Contact
Stefanie Korsgaard Körner, MD, PhD-student
Clinicaltrial.org: NCT04321707
Treatment
MOSAIC - Modified Urinary Conduit to Lower Strictures After Radical Cystectomy- DaBlaCa 16
Title: MOSAIC – Modified Urinary Conduit to Lower Strictures After Radical Cystectomy – DaBlaCa – 16
Aim
To compare the rate of left sided ureteral strictures after radical cystectomy between the modified retrosigmoid conduit (Intervention Group) and the standard urinary conduit (Control group).
Number of patients
300
Methods
This project is a randomized controlled trial where patients undergoing cystectomy are randomized between the modified conduit and the conventional ileal conduit ‘ad modum Bricker‘. Primary endpoint is the rate of ureterenteric strictures, and secondary endpoint is both surgical complications and renal function within 24 months.
Status
Ongoing
Sites
Department of Urology, Aalborg University Hospital
Department of Urology, Aarhus University Hospital
Department of Urology, Odense University Hospital
Department of Urology, Rigshospitalet
Department of Urology, Herlev and Gentofte Hospital
Contact:
Phd student: Simone Buchardt Brandt, email: simbra@rm.dk
Clinicaltrial.gov: NCT04391790
Survival of patients with muscle invasive bladder cancer after the implementation of neoadjuvant chemotherapy in the period 2010-2015 - DaBlaCa 17
Title: DaBlaCa-17: Survival of patients with muscle invasive bladder cancer after the implementation of neoadjuvant chemotherapy in the period 2010-2015.
Aim
This study will evaluate whether the implementation of neoadjuvant chemotherapy in 2013 has added a survival benefit to patients with muscle invasive bladder cancer treated with neoadjuvant chemotherapy on a national basis.
Number of patients:
Methods
A retrospective study with data collected from the five urological centers performing cystectomy in Denmark.
Status
ongoing
Sites
Aarhus University Hospital, Aalborg University Hospital, Odense University Hospital, Rigshospitalet, Copenhagen, Herlev Hospital.
Contacts
Stefanie Korsgaard Körner, MD, PhD-student
Influence of Hormone Treatment in Radiation Therapy for Bladder Cancer - DaBlaCa 18
Title: Influence of Hormone Treatment in Radiation Therapy for Bladder Cancer – DaBlaCa 18
Aim
To determine if concomitant treatment with androgen deprivation therapy during radiation therapy (RT) for bladder cancer is associated with a lower risk of radiation side effects such as fibrosis, decreased bladder compliance and overall decreased quality of life.
Number of patients
63
Methods
Prospective, observational cohort study. Patients are included when diagnosed with muscle invasive bladder cancer and are scheduled to receive radiation therapy with or without concomitant treatment with androgen deprivation therapy. Before and during the RT patients will receive questionnaires regarding side effects. After completion of the RT, they will undergo a cystoscopy with biopsy in which degree of fibrosis will be determined. Finally, they will complete questionnaires and urodynamic examination 3 months after completing RT and again 12 months later.
Status
Ongoing
Sites:
- Department of Urology, Aarhus University Hospital
- Department of Urology, Odense University Hospital
Contacts: PhD Student Josephine Hyldgaard, email: josehyld@rm.dk
Clinicaltrial.gov: NCT04282876
EGIAES
Title: Endo-GIA Versus Endowrist Stapler In Intracorporeal Urinary Diversion In Robotic Assisted Radical Cystectomy – EGIAES
Cystectomy with urinary diversion is the standard treatment of muscle invasive and high risk non-muscle invasive bladder cancer. During cystectomy, a urinary diversion is constructed from a bowel segment. Restoration of the intestinal continuity is therefore an obligate part of the procedure.
In Denmark, approximately 400 radical cystectomies are performed yearly with the majority of procedures performed as a laparoscopic robot assisted procedure. This includes urinary diversion by means of intracorporeal procedure.
During current standard intracorporeal urinary diversion, an Endo-GIA stapler is handled by the assisting surgeon and not by the main surgeon as the Endo-GIA stapler is not integrated into the robot.
The traditional Endo-GIA anastomosis is made as a side-by-side anastomosis with two 60 mm magazines: one for the side-to-side anastomosis and one for closing the end.
Any reduction in the lumen of the anastomosis will clinically affect post-operative bowel function. It is known that at all cystectomy patients have intestinal paralysis / lack of normal bowel function in the first days postoperatively. It is thus plausible that a wider anastomosis will be able to reduce the duration of this in favor of the patient’s post-operative nutrition, postoperative length of stay and convalescence.
A stapler integrated in the robot (Endowrist stapler from Intuitive) is available. This has several advantages: it is operated by the robotic surgeon and not by the assistant, it is more flexible, and faster mobility. These advantages provide the possibility of precisely removing a minimal intestinal segment by the final transverse stapling. The biggest disadvantage of the robot-operated Endowrist staple is that it is not available in a 60 mm version but only in 45 mm, thus giving only an anastomosis of approximately the same lumen as using a 60 mm Endo-GIA staple but not better.
An opportunity to make a more spacious anastomosis would be to “prolong” the longitudinal stapling as to the side-to-side anastomosis between the intestinal segments. This requires precise and coordinated handling of bowel graspers and staplers to make a complete elimination of the risk of anastomosis leakage, which in this respect is an advantage of robot-operated staples with the Endowrist stapler rather than an assistant handled stapler with Endo-GIA.
Both Endo-GIA and Endowrist stapler are approved for clinical use according to the procedures described.
Contact
Professor Jørgen Bjerggaard Jensent, email: bjerregaard@skejby.rm.dk
Follow-Up
TOMBOLA - Treatment Of Metastatic Bladder cancer at the time Of biochemical reLApse following radical cystectomy - DaBlaCa 14
Title: TOMBOLA – Treatment Of Metastatic Bladder Cancer at the Time Of Biochemical reLApse Following Radical Cystectomy – DaBlaCa 14
Aim
The study aim at investigate the response rate and oncological outcome of early systemic immunotherapy treatment with the PDL-1 inhibitor Atezolizumab. The study drug is administered at the time of biochemical relapse e.g. circulating tumor DNA (ctDNA positive) in patients who have undergone radical cystectomy due to muscle invasive bladder cancer. Biomarkers that predict response to systemic immunotherapy will be identified by comprehensive multi-omics analysis of primary tumors and metastatic lesions. Furthermore, the trial aim to determine if ctDNA levels during therapy can be used as a biomarker for early indication of therapy response.
Number of patients
282 patients
Methods
The study is conducted as a Single Country, Open-label, Single-arm, Non-randomized, Phase II study where intervention is given based on an experimental design.
Sensitive molecular techniques for detection of tumor DNA in the blood is performed to identify patients with early metastatic disease.
The study drug will be administered according to current recommendations as systemic treatment every third week for a maximum of 12 months or until progression. Treatment will be initiated within 28 days of diagnosis of ctDNA relapse.
Status: Open for inclusion at both main- and sub sites.
Sites: Dept. Of Urology and Oncology from:
- Aarhus University Hospital
- Herlev Hospital
- Rigshospitalet
- Odense University Hospital
- Aalborg University Hospital
Contacts
National Trial Coordinator, Tine Christiansen, email: tinechti@rm.dk, Phone: + 45 30 91 54 59
Sponsor-Investigator: Jørgen Bjerggaard Jensen. E-mail: bjerggaard@skejby.rm.dk
Clinicaltrial.gov: NCT04138628
Nordic cystectomy biomarker validation study - NorCys
Title: Nordic cystectomy biomarker validation study – NorCys
Muscle-invasive bladder cancer (BC) is an aggressive malignancy and radical cystectomy (RC) with pelvic lymph node dissection (PLND) is the gold standard of therapy. In addition to surgery, neoadjuvant chemotherapy (NAC) is recommended for patients who are fit for cisplatin-based chemotherapy based on results from prospective trials. In the Nordic countries approximately 7.200 new BC cases are diagnosed annually and mortality is 2.100 cases per year. We estimate that about 1.200 RCs are performed due to MIBC in Nordic countries per year.
Currently the tools for estimating prognosis and risks are insufficient and improved ones are needed.
Aims
The rationale for the study is to gather, in a prospective, multi-institutional and international design, data of patients undergoing RC for MIBC in Nordic countries. The collected data will be used with the aim of validating existing prediction tools and discover novel tools for
1) prediction of morbidity related to RC.
2) prediction of oncological outcome after RC.
3) prediction of response to NAC prior to RC.
The hypothesis is that with this study we can meet the abovementioned aims and develop a well-documented model for prediction of individual patient outcome regarding RC.
Sites
This study is in collaboration with all the Nordic countries, through the Nordic Urothelial Cancer Research Group, led by Peter Boström, Cheif at the Department of Urology at Turku University Hospital, Finland.
Read more: http://north-reg.nu/
Contact
PhD student Simone Buchardt Brandt, email: simbra@rm.dk
Professor Jørgen Bjerggaard Jensen
Clinicaltrial.gov: NCT04523025, NCT04537221, NCT04523038
Uromonitor - Surveillance of low-grade non-muscle invasive bladder tumours using Uromonitor
Title: Surveillance of low-grade non-muscle invasive bladder tumours using Uromonitor
Aim
The’ study aim at evaluating the potential clinical impact of the ‘Uromonitor-test’ in the surveillance program of Low Grade NMIBC. The study will evaluate if the non-invasive surveillance test can reduce the number of flexible cystoscopies, without increasing the risk of progression.
Number of patients
160 subjects
Methods
The study is designed as a multicenter observational clinical trial. Patients scheduled for follow-up cystoscopy due to previous Low Grade NMIBC within the first 2 years from diagnosis, where no recurrence is found at the current cystoscopy, will be offered inclusion in the study.
At each visit (baseline, 4, 8, 12, 16, 20 and 24 months from inclusion) the study subjects will contribute with a urine sample of 20 ml. The specimen will analysed according to protocol using the ‘Uromonitor’ test.
Status
Open for inclusion
Sites
Sponsor-site: Department of Urology, Zealand University Hospital, Roskilde.
Sub-site: Bladder Cancer Research team, Dept.of Urology
Contacts
Sponsor- investigator: Nessn H. e. Azawi, MD. Ph.D. nesa@regionsjaelland.dk
Sub-Investigator: Thomas Karmark Dreyer, MD, thomas.dreyer@rm.dk
Principal Investigator: Jørgen Bjerggard Jensen, MD, DMSc, Bjerggaard@skejby.rm.dk
PAGER
Klinisk relevante biomarkører til forudsigelse af sygdomsforløb og terapirespons hos patienter med blærekræft er stadig ikke blevet identificeret trods en intensiv forskningsindsats. Nye teknologiske fremskridt indenfor kortlægningen af vores arvemasse (genom) har gjort det muligt at identificere genomiske ændringer, som er specifikke for den enkelte patients tumor. Det er derfor nu muligt at bruge disse teknologiske fremskridt til bedre kontrol af den enkelte patients sygdom.
Dette projekts formål er vha. ”Next Generation Sequencing” (NGS) at identificere mutationer og genomiske ændringer i genomet i tumorer fra patienter med blærekræft. Ændringerne vil blive anvendt som patient-specifikke markører til at følge sygdomsudviklingen over tid. De specifikke formål med projektet er:
Identifikation af specifikke markører til test af urinprøver hos patienter med ikke muskelinvasiv blæretumor som følges med cystoskopiundersøgelser
Identifikation af specifikke markører til test af blodprøver hos patienter med muskelinvasiv blærekræft behandlet med neoadjuvant kemoterapi og efterfølgende cystektomi
Identifikation af specifikke markører til test af blodprøver hos patienter med avanceret muskelinvasiv blærekræft behandlet med kemoterapi
Med dette projekt vil vi belyse, om det er muligt at identificere sygdomstilbagefald og sygdomsforværring på et tidligere tidspunkt vha. urin og blodprøver, og dermed forbedre overlevelsen for patienter med blærekræft. Yderligere vil vi undersøge om vi, vha. personlige biomarkører, kan undgå gentagne kikkertundersøgelser af blæren, og dermed spare patienterne for smerter og bivirkninger ved dette, samt spare store udgifter forbundet med de nuværende kontrolundersøgelser. Desuden vil vi med projektet belyse, om vi kan identificere spredning af sygdommen tidligere. Dermed vil man kunne igangsætte kemoterapi på et tidligere tidspunkt, hvilket ultimativt vil kunne forbedre patienternes overlevelse. Endelig vil måling af kemoterapi-effektivitet ultimativt sikre, at patienterne tilbydes den relevante behandling.
Contact
Professor Jørgen Bjerggaard Jensen and clinical trial coordinator Anna Munk
Biorepository
Bio – and Genome Bank Denmark
Title: Bio – and Genome Bank Denmark
Aim
The biorepository aims specifically at creating a solid foundation for research in bladder cancer to develop new methods for optimal diagnose and personalized treatment.
Number of patients
>5600 participating patients from the Central Denmark Region
Methods
Denmark has a long tradition of collection biological specimen from various disease categories. In patients diagnosed with bladder cancer the biological specimen has been collected prospectively since 1994. The bladder cancer biorepository represents samples from disease categories as Carcinoma In Situ, pTa LG and HG, pT1-4. The collection of blood, urine and tissue from patient with NMIBC and MIBC has been ongoing since 1994. Patients are included in the biobank at the time of diagnosis, and samples are collected at each hospital visit throughout the course of treatment and follow-up visits. When processed the material is shipped to Department of Molecular Medicine at Aarhus University Hospital. The large cohort represents a unique and valuable material for researchers and clinicians in their investigation of expected disease progression, probability of recurrence, response to treatment ect. The repository contains detail information on clinical characteristics, types of treatment, pathological reports ect.
Sampling events for patients included in the bladder cancer biorepository at Aarhus University Hospital:
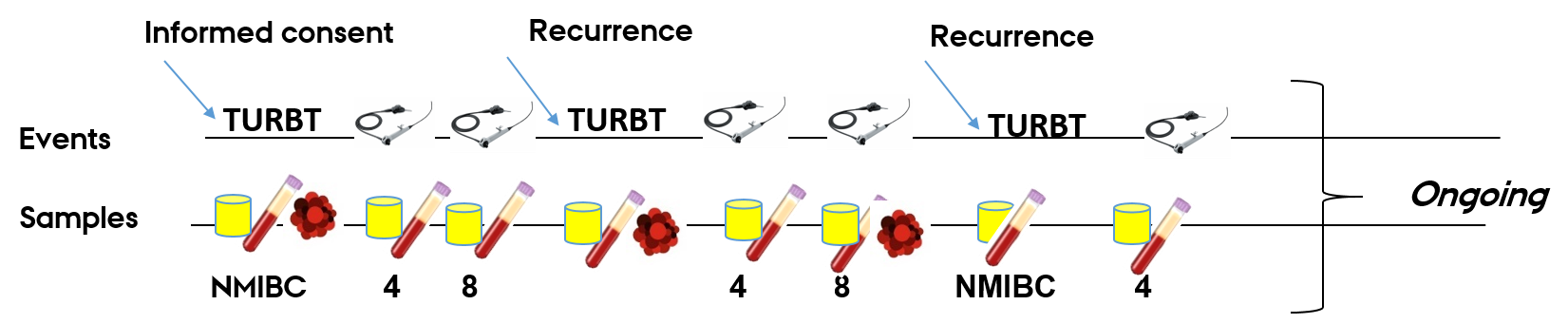
Samples per visit:
Liquid biopsies: 2x 10 mL. lavender top EDTA, 2x 10 mL serum separation tube, 2 x 4,5 mL urine and 1x tumor tissue biopsy.
Status
- 1-5 tumor tissue samples from each patient
- > 13000 vials with full blood
- > 3300 vials buffy coat
- > 36000 urine samples
Sites
Aarhus University Hospital and Regional Hospital West Jutland
Contacts
Medical laboratory technologists
Jameela Safi: jamesafi@rm.dk
Research coordinator for bladder cancer projects
Clinical Trial Coordinators
Vibeke Juul Morrison
Tlf: +45 24 44 23 54
Email: vimorr@rm.dk
Tine Maria Bonde Christiansen
Tlf: +45 30 91 54 59
Email: tinechti@rm.dk
Dorthe Sønderborg Welch
Tlf: +45 30 91 56 32
Email: dowelc@rm.dk
PhD students
Postdoc
Research assistant
Project biomedical laboratory scientists
Johanna le Fevre
Tlf: +45 30 91 54 20
Email: johlef@rm.dk
Gamze Ipek Alkan
Tlf: +45 24 60 82 92
Email: gamalk@rm.dk
Derya Ayyildez
Tlf: +45 24 60 82 92
Email: deroez@rm.dk
Jameela Safi
Email: jamesafi@rm.dk
Currently on maternity leave
Professor secretary
Johanne Frost Lunding
Tlf: +45 91 30 55 25
Email: JOHALN@rm.dk
Mette Studstrup
Tlf: +45 30 91 55 25
Email: MESTUD@rm.dk
Currently on maternity leave
